First, the meaning of welding
Soldering is to use a material with a lower melting point than the metal to be welded and heat it together with the metal to be welded. Under the condition that the metal to be welded does not melt, the molten solder wets the metal surface and forms an alloy layer on the contact surface to achieve a firm connection. the process of.
Why does the solder wet the metal being soldered during the soldering process? How can I get a reliable connection? Through the analysis of the welding principle, a preliminary understanding can be obtained.
The formation of a solder joint goes through three stages of changes: 1. The wetting stage of the molten solder on the surface of the metal to be welded; 2. The expansion stage of the molten solder on the surface of the metal to be welded; 3. The molten solder penetrates the weld through capillary action , forming an alloy layer on the contact surface with the metal to be welded. Among them, wetting is the most important stage, without wetting, welding cannot proceed.
2. Wetting effect of welding
When any liquid and solid come into contact, there will be different degrees of wetting. During soldering, molten solder (liquid) adheres to various metal surfaces to varying degrees, and can expand to varying degrees. This adhesion is wetting. The stronger the wetting, the larger the expansion surface, the better the wetting, and conversely, the wetting is not good or not at all.
The reason for the difference in the degree of wetting is that the mutual attraction (adhesion) between the liquid part (molten solder) and the solid molecule (the metal to be soldered) is greater or less than the mutual attraction (surface tension) between the liquid molecules ) determined, namely:
Adhesion > surface tension, wetting;
Adhesion < surface tension, no wetting.
According to the above principle, reducing the surface tension of the molten solder during soldering can improve the wetting ability of the solder to the metal to be soldered. The most effective way to reduce the surface tension of the solder is to use flux when soldering.
In order for the solder to quickly wet the metal to be soldered, direct metal-to-metal contact must be achieved, that is, the contact surface between the solder and the metal to be soldered must be clean, and any contamination will prevent wetting and metal compound formation. Therefore, maintaining clean contact surfaces is a must for wetting. However, there are always oxides and oil stains on the metal surface, so the metal surface to be welded must be cleaned before welding.
3. Formation of solder joints
3.1 The force formed by the solder joint
The formation of a solder joint is the result of the combined action of multiple forces. Apply a layer of flux on a clean copper plate and place a certain amount of solder on it, then heat the copper plate to a specified temperature, and the solder melts to form the shape shown below.
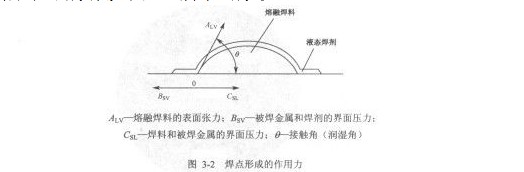

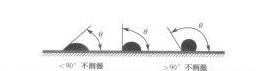
Figure 3-3
(Figure 3-2), it can be seen that through the size of the contact angle, the wettability of the solder to the metal to be soldered can be measured, as shown in Figure 3.3.
When θ<90°, COSθ>0, wetting
When θ>90°, COSθ <0, no wetting
COSθ→1 when θ→0°, completely wet
COSθ→-1 when θ→180°, not wet at all
Generally speaking, θ=20°~30°, which is good wetting and qualified solder joints.
It can be seen from the above that the quality of solder joint formation depends on the properties of the solder and flux, as well as the surface state of the metal to be soldered, as well as the welding process conditions and operation methods.
3.2 Formation of solder joints (alloy layer) - intermetallic diffusion
When the molten material wets the metal to be welded, intermetallic diffusion occurs, thereby forming an alloy layer on the metal contact surface to achieve the final purpose of welding.
The interior of any metal is not completely dense, and there is always a certain number of gaps or holes inside the lattice or at the interface of the lattice. Under normal circumstances, metal atoms in the crystal lattice can perform continuous thermal motion centered at their equilibrium position. This motion increases with temperature, and its frequency and energy gradually increase. When sufficient energy and temperature are reached , some atoms will overcome the bondage of surrounding atoms and break away from the original occupied position. This phenomenon is diffusion. For example, when copper and copper alloys are welded, under certain process conditions, the tin atoms in the solder and the copper atoms of the metal to be welded will diffuse to the metal contact surface to form copper-tin alloys (Cu5Sn6, Cu3Sn).
The diffusion speed and amount of diffusion between metals are closely related to temperature and time. Therefore, in welding technology, processes such as welding temperature and welding time are important conditions to ensure reliable connection of welding quality.
In addition, whether the alloy layer can be formed during welding also depends on the affinity between the metal to be welded and the solder. If the affinity is large, a metal compound (alloy layer) will be formed; if the affinity is small, a solid solution will be formed; if the affinity is particularly small, a mixture will be formed. This shows that the wetting of the soldered metal by the solder is related to the properties of the soldered metal itself. Among the electronic conductive materials, Cu, Au, Ag and other materials and coatings are mostly used to improve the wetting ability of the solder to the soldered metal.
Fourth, the countermeasures to improve the welding quality
According to the above analysis of the welding mechanism, we know that the welding process of electronic products is a complex physical and chemical change process, and the formation of solder joints is the result of comprehensive forces. Therefore, the countermeasures to improve the welding quality are:
- A clean contact surface must be maintained;
- Find a way to reduce the surface tension of the solder;
- To maintain a certain welding temperature and welding time;
- To understand the surface properties of the metal being welded.
-

-


 Language
Language  中文简体
中文简体 English
English


 Address:A No.13 Twenty-third Road, South Economic and Technological Development Zone, Shenyang City, Liaoning Province.
Address:A No.13 Twenty-third Road, South Economic and Technological Development Zone, Shenyang City, Liaoning Province. Phone:13898848281
Phone:13898848281 Landline:024-25799905
Landline:024-25799905 Fax:024-25799905
Fax:024-25799905 E-mail:jyjg2018@163.com
E-mail:jyjg2018@163.com